The Medical Packaging Industry must adhere to strict safety guidelines and standards for the safety of patients. A compromised or contaminated product could be the difference between life or death for a patient and a massive liability for the medical packaging company. Under these tough safety standards, the Medical Packaging Industry has found ways to innovate and create a safer packaging process and product. The medical packaging market’s value is expected to reach almost $42 billion dollars by 2026 but they’re not immune to current packaging trends. Pouches have taken the consumer packaging industry by storm and now this flexible packaging option is making its way into the Medical Packaging sector. One innovation that has revolutionized the Medical Packaging Industry is the Tyvek pouch packaging for medical devices and instruments.
What is Tyvek?
Tyvek is a sturdy, water-repellent, lightweight material used in a wide variety of applications, ranging from wrapping homes to medical packaging and PPE. Its water and chemical resistance qualities create a microbial barrier that protects against the elements and more. Tyvek’s water resistance, durability, and tear-resistant qualities make it highly regarded in the packaging industry. For example, the United States Postal Service (USPS) uses Tyvek to make envelopes that protect documents against the elements and harsh conditions faced by mail as it moves through the mail system for delivery.
In Medical Packaging, Tyvek is specifically used to package sterilized medical devices and equipment. Tyvek pouches are common packaging for single-use medical instruments. It is the ideal material as Tyvek withstands most of the commonly used sterilization methods, including, Ethylene Oxide, Radiation, low-temperature oxidative, and more. The material’s durability and tear resistance reduce the likelihood of punctures and prevent contamination of the sterilized product.
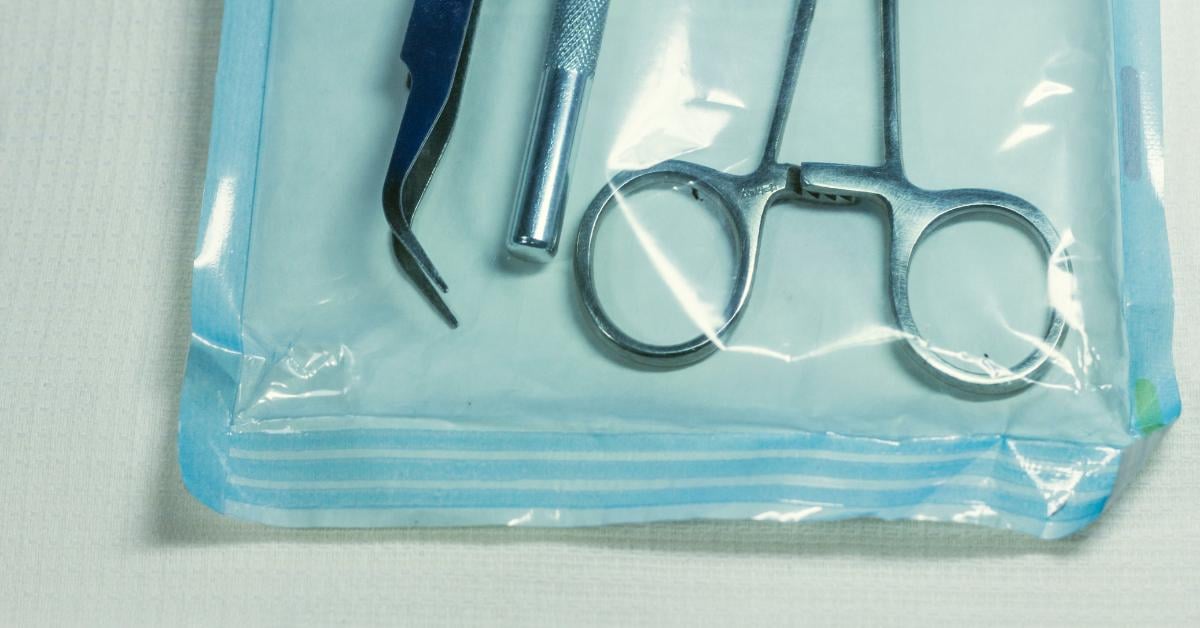
Printing on Tyvek
In Medical Packaging, Unique Device Identification (UDI) requirements are put in place to track products and packaging through the manufacturing process. UDI requirements include such as barcodes, serial numbers, and more to hold identification information. In Medical Packaging, tracking and tracing provide transparency in the packaging process. UDI and serialization are essential for the identification and tracing of incidents and defects. Businesses can follow their product from production to packaging to distribution and even to customer purchases with UDI codes. Defects can be traced to distribution locations and even specific production lines.
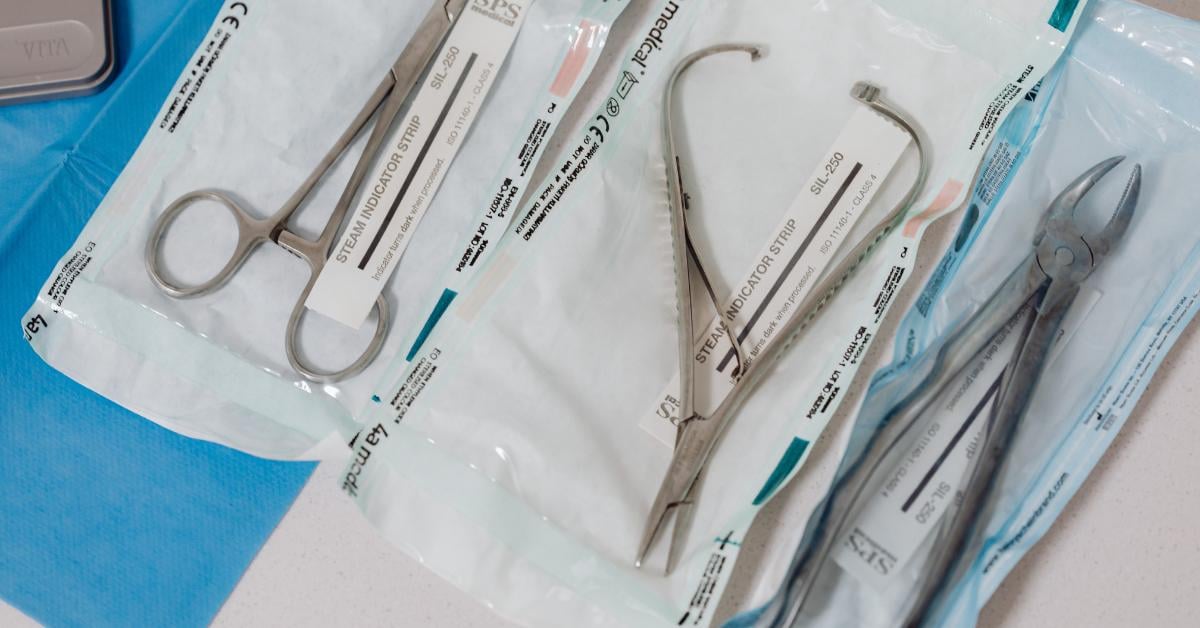
Printing on Tyvek can be complex due to its water-repellent nature with many inks unable to stick to the nonporous surface. Smudging or deterioration of the print job can occur if the ink is not dry before moving to the next step of the packaging process. It is crucial that the UDI barcodes and serial numbers remain clear for scanning and tracking purposes. It is recommended to use a Thermal Transfer Print System to print onto Tyvek as it does not use water-based inks.
Thermal Transfer Print System
MFT Automation’s Print System has a modular design that allows for customizable print engine integration. The Thermal Transfer print engine is the best option for printing on Tyvek. Thermal transfer printing is when a material is applied to paper by melting a coating ribbon that glues the ink to the material. Unlike water-based inks, Thermal Transfer printing bonds the ink to the surface, avoiding the issues caused by Tyvek's water-resistant qualities and creating high-quality, long-lasting prints.
To accommodate the strict sanitation standards of the medical device packaging industry, the system can be outfitted with Hygienic Washdown equipment that is specifically designed for environments that need to be kept sanitary. Made with Type 304 Stainless Steel, the material of choice for medical packaging applications, the equipment is designed to be repetitively and aggressively cleaned every day to adhere to safety regulations and guidelines.
See the system in action!
Your Automation Solutions Partner
MFT Automation has been working with customers to find the best solution for their automation challenges for over 25 years. We’re your one-stop shop for automation solutions. Our process starts with our engineering-oriented and customer-driven approach. Our incredible teams of Mechanical, Electrical, and Software Engineers collaborate with customers to completely understand each customer’s unique application. We develop and design high-performance automation systems based on the customer’s needs. We’re proud to manufacture our automation equipment in-house. Our excellent production teams work in a machine shop with modern CNC and lathe machine tools allowing us to offer highly customized equipment and systems. Our Integration team specializes in testing and putting the finishing touches on the system. After integration and installation, we offer abundant after-market services, including training, convenient spare parts ordering, and exceptional customer service.




